
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
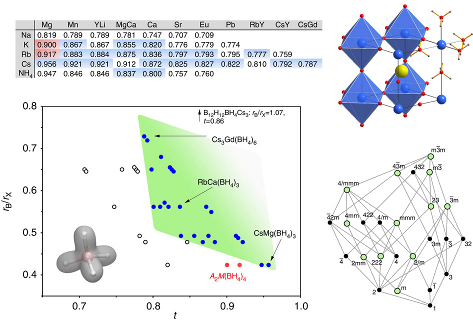
Perovskite materials host an incredible variety of functionalities. Although the lightest element, hydrogen, is rarely encountered in oxide perovskite lattices, it was recently observed as the hydride anion H−, substituting for the oxide anion in BaTiO3. Here we present a series of 30 new complex hydride perovskite-type materials, based on the non-spherical tetrahydroborate anion BH4− and new synthesis protocols involving rare-earth elements. Photophysical, electronic and hydrogen storage properties are discussed, along with counterintuitive trends in structural behaviour. The electronic structure is investigated theoretically with density functional theory solid-state calculations. BH4-specific anion dynamics are introduced to perovskites, mediating mechanisms that freeze lattice instabilities and generate supercells of up to 16 × the unit cell volume in AB(BH4)3. In this view, homopolar hydridic di-hydrogen contacts arise as a potential tool with which to tailor crystal symmetries, thus merging concepts of molecular chemistry with ceramic-like host lattices. Furthermore, anion mixing BH4−←X− (X−=Cl−, Br−, I−) provides a link to the known ABX3 halides. | ||||||||
|
||||||||
The crystal structure of the third polymorph of dibenzylsquaramide (Portell, A. et al., 2009), (fig. 1) has been determined from laboratory X-ray powder diffraction data by means of direct space methods using the computing program FOX. (Favre-Nicolin and Černý, 2002) The structure resolution has not been straightforward due to several difficulties on the indexing process and in the space group assignment. The asymmetric unit contains two different conformers, which has implied an additional difficulty during the Rietveld (Rietveld, 1969) refinement. All these issues together with particular structural features of disquaramides are discussed. | ||||||||
 |
|
|||||||
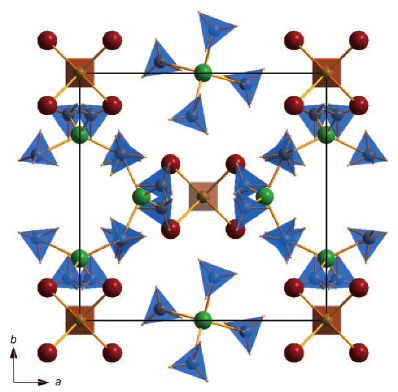
Four novel bimetallic borohydrides have been discovered, K2M(BH4)4 (M = Mg or Mn), K3Mg(BH4)5, and KMn(BH4)3, and are carefully investigated structurally as well as regarding their decomposition reaction mechanism by means of in situ synchrotron radiation powder X-ray diffraction (SR-PXD), vibrational spectroscopies (Raman and IR), thermal analysis (TGA and DTA), and ab initio density functional theory (DFT) calculations. Mechano-chemical synthesis (ball-milling) using the reactants KBH4, α-Mg(BH4)2, and α-Mn(BH4)2 ensures chlorine-free reaction products. A detailed structural analysis reveals significant similarities as well as surprising differences among the two isomorphs K2M(BH4)4, most importantly concerning the extent to which the complex anion [M(BH4)4]2– is isolated in the structure. Anisotropic thermal expansion and an increase in symmetry at high temperatures in K3Mg(BH4)5 is ascribed to the motion of BH4 groups inducing hydrogen repulsive effects, and the dynamics of K3Mg(BH4)5 are investigated. Decomposition in the manganese system proceeds via the formation of KMn(BH4)3, the first perovkite type borohydride reported to date. | ||||||||
|
 |
|||||||
The new double-cation Al-Li-borohydride is an attractive candidate material for hydrogen storage due to a very low hydrogen desorption temperature (~70 °C) combined with a high hydrogen density (17.2 wt %). It was synthesised by high-energy ball milling of AlCl3 and LiBH4. The structure of the compound was determined from image-plate synchrotron powder diffraction supported by DFT calculations. The material shows a unique 3D framework structure within the borohydrides (space group=P-43n, a=11.3640(3) Å). The unexpected composition Al3Li4(BH4)13 can be rationalized on the basis of a complex cation [(BH4)Li4]3+ and a complex anion [Al(BH4)4]-. The refinements from synchrotron powder diffraction of different samples revealed the presence of limited amounts of chloride ions replacing the borohydride on one site. In situ Raman spectroscopy, differential scanning calorimetry (DSC), thermogravimetry (TG) and thermal desorption measurements were used to study the decomposition pathway of the compound. Al-Li-borohydride decomposes at ~70 °C, forming LiBH4. The high mass loss of about 20 % during the decomposition indicates the release of not only hydrogen but also diborane. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017
